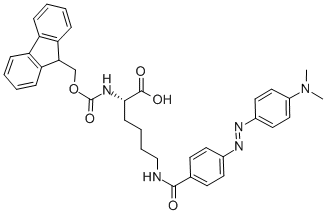
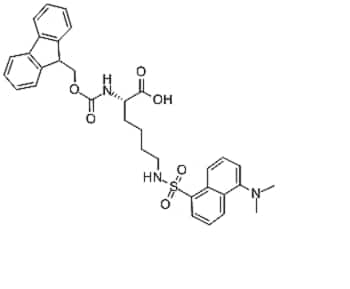
Showing 33–48 of 90 results

"Orthogonally-protected building block for the synthesis of branched and cyclic peptides and peptides modified at the Lys side chain. The Alloc group can be selectively removed in the presence of standard Fmoc- and t-butyl-based protecting groups by treatment with Pd(Ph3P)4/CHCl3/AcOH/NMM. Fmoc-Lys(Alloc)-OH, N-alpha-Fmoc-N-epsilon-allyloxycarbonyl-L-lysine Fmoc-Lys(Aloc)-OH is used where the lysine sidechain must be selectively removed. The Aloc protecting group can be removed with Pd(0) catalyst and a proton source."

Fmoc-Lys(Boc)-OH has emerged as the standard Fmoc-Lys derivative used in peptide synthesis. The Boc group on the side chain is stable under basic conditions and remains in place even through many Fmoc deprotection cycles. The Boc group of Fmoc-Lys(Boc)-OH is readily removed with trifluoroacetic acid (TFA) during cleavage of the peptide product from Wang resin or Rink amide resin. The standard reagent for coupling lysine into peptide sequences.