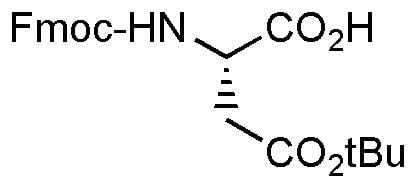
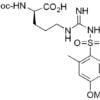
Fmoc-Asp(OBut)-OH – standard Fmoc-protected derivative of aspartic acid
Purchase Fmoc-Asp(OBut)-OH; Fmoc-L-Aspartic acid beta-tert-butyl ester; 71989-14-5
View Safety Data Sheet (SDS)
| 5g | $20.00 |
| 25g | $35.00 |
| 100g | $105.00 |
Fmoc-Asp(OtBu)-OH is the standard Fmoc-protected derivative of aspartic acid used in peptide synthesis. The t-butyl ester of the side chain is readily hydrolyzed in the same conditions used to cleave peptides from Wang resin or Rink amide resin. The t-butyl cations formed in the hydrolysis of the esters can produce byproducts by reattaching to nucleophilic residues in the peptide, so nucleophilic scavengers such as thiophenol and anisole should be added to the cleavage mixture when Fmoc-Asp(OtBu)-OH is utilized. See prices below.
| Catalog Number | FD2192 |
| PubChem CID | 2724635 |
| Canonical SMILES | CC(C)(C)OC(=O)CC(NC(=O)OCC1c2ccccc2-c2ccccc21)C(=O)O |
| Isomeric SMILES | CC(C)(C)OC(=O)C[C@@H](C(=O)O)NC(=O)OCC1C2=CC=CC=C2C3=CC=CC=C13 |
| Instance Of | type of chemical entity |
| CAS Registry Number | 71989-14-5 |
| Chemical Formula | C₂₃H₂₅NO₆ |
| UniChem Compound ID | 24152872 |
| InChI | InChI=1S/C23H25NO6/c1-23(2,3)30-20(25)12-19(21(26)27)24-22(28)29-13-18-16-10-6-4-8-14(16)15-9-5-7-11-17(15)18/h4-11,18-19H,12-13H2,1-3H3,(H,24,28)(H,26,27)/t19-/m0/s1 |
| InChIKey | FODJWPHPWBKDON-IBGZPJMESA-N |
| Mass | 411.16818752000006 |
| Subclass Of | chemical compound |
Fmoc-Asp(OBut)-OH – standard Fmoc-protected derivative of aspartic acid
Fmoc-Asp(OBut)-OH – standard Fmoc-protected derivative of aspartic acid Related Compounds with Annotation
Fmoc-Asp(OBut)-OH – standard Fmoc-protected derivative of aspartic acid Depositor-Supplied Synonyms
Fmoc-Asp(OBut)-OH – standard Fmoc-protected derivative of aspartic acid R3D Conformer
Fmoc-Asp(OBut)-OH – standard Fmoc-protected derivative of aspartic acid GHS Classification
Buy Fmoc-Asp(OBut)-OH; Fmoc-L-Aspartic acid beta-tert-butyl ester; 71989-14-5 online.